Atomic Structure
What Is An Atom?
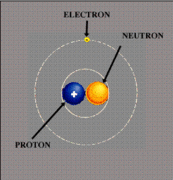
Atoms are a basic unit of matter, composed of of three types of particles: protons, neutrons, and electrons. Protons are the positive charge, electrons are the negative charge, and neutrons are neutral. If an atom has a positive or negative charge, it is called an ion. A group of atoms can bond together to form a molecule. Atoms are particles of elements, which cannot be broken down furthur unless the chemical nature of the substance is changed.
Protons
Protons have a relative mass of 1 and a relative charge of +1. The protons of an atom are found in the nucleus. The number of protons can be used to determine the atomic number of an atom.
Electrons
Electrons have a relative mass of 1/1836 and a relative charge of -1. The electrons of an atom are found in the energy levels around the nucleus, varying in distances. The number of the electrons is the same as the number of protons in an atom, which also equals the atomic number.
Neutrons
Neutrons have a relative mass of 1 and a relative charge of 0. The neutrons are found in the nucleus of an atom, along witht the protons. The number of protons in an atom added to the number of neutrons in an atoms is equal to the mass number of the atom.